Anxiety Disorders Show Changes In Cerebral Hemisphere Symmetry In Which Scan?
Introduction
Stroke is a major reason for permanent disability and a leading crusade of death, which affects public health and results in large costs (Katan & Luft, 2018). Diagnostic imaging plays a primal office in stroke management with Computed Tomography (CT) and/or Magnetic Resonance (MR) imaging employed to make the diagnosis and therapeutic decisions. Noncontrast Computed Tomography (NCCT) remains the start-line diagnosis for emergency evaluation of acute stroke because it is fast, widely available, price-efficient, and reliably rules out hemorrhage (Lövblad & Baird, 2010). Early ischemic changes in NCCT scans are characterized by several features including the presence of hypodensity within the infarcted region, loss of distinction betwixt grayness and white affair, diminishing of the basal ganglia contrast, sulcal effacement, ventricular narrowing, disappearing of insular ribbon, and/or a center cerebral avenue hyperdensity sign. We also accept observed the presence of the hyperdense posterior cerebral artery sign (Ambrosius et al., 2011). NCCT, however, has poor sensitivity, particularly in the first few hours, equally astute ischemic changes on NCCT are subtle and often do not prove infarct until 12–24 h after stroke onset (James et al., 2006). When compared to MR, this sensitivity is 25% in NCCT versus 86% in MR; however, within the first three h, it is lowered to 7% for NCCT and 46% for MR (Chalela et al., 2007).
The stroke-caused changes in a NCCT prototype may be as well imperceptible to the homo eye to be detected, peculiarly in the hyperacute phase, therefore their computer-assisted processing and assay could assist in enhancing and expediting the scan reading. In general, there are three approaches to delineate lesions in brain images: manual, semi-automatic, and fully automated, and a manual tracing past trained professionals remains the golden standard (Fiez, Damasio & Grabowski, 2000; Wilke et al., 2011). Specially for follow-upwardly scans, the reliable and reproducible lesion segmentation is of high interest, as the lesion book is one of import imaging end-point for clinical trials. A manual delineation of a encephalon lesion is laborious and fourth dimension-consuming, and information technology requires substantially more than man input compared to the automatic arroyo (e.g., a few hours versus one minute as compared by Wilke et al. (2011)). Therefore, automating the detection and segmentation of ischemic infarcts is disquisitional, especially, every bit the time window to treat stroke is 3-iv.5 h for intravenous thrombolysis (Hacke et al., 2008). Moreover, a transmission approach results in variability beyond operators, because there is ofttimes no clear cutoff between lesioned and non-lesioned tissues, especially, at the brain's borders and effectually the cerebral ventricles as well as it unremarkably does not detect inevitable stroke-induced changes taking place outside the lesion (Fiez, Damasio & Grabowski, 2000). Semi-automated methods combine advantages of a fully automated abnormality detection with manual editing of the lesion enabling the operator to finalize its location and extent.
A calculator-assisted approach, even without applying fully automated methods, has been employed in stroke paradigm management. For instance, Mainali, Wahba & Elijovich (2014) demonstrated that a simple transformation of image brightness and contrast, by changing the window center and width level in standard windows and introducing improved stroke windows, significantly improves detection (from eighteen% to 70%) of early ischemic changes.
A popular approach for stroke detection is to apply the ASPECTS score. The ASPECTS (meaning the Alberta Stroke Programme Early CT Score) aims to systemize the detection and reporting of ischemic stroke by visually identifying an ischemic hypodensity on the middle cerebral avenue (MCA) territory subdivided into ten regions that are located on two unlike axial CT slices (Barber et al., 2000). In order to automate this visual approach, Kuang et al. (2019) proposed a method based on texture features extracted from each ASPECTS region to train a random forest classifier. This automated arroyo tested on 100 patients showed a reasonable ability to determine the ASPECTS.
Numerous methods have been adult for semi-automated and automated stroke lesion detection and delineation, generally in MR images; however, simply a few approaches have been proposed for detecting stroke lesions in NCCT scans (Rekik et al., 2012). Moreover, in comparison to the development of hemorrhagic stroke processing methods that of ischemic stroke detection is given less attention because of its more demanding nature (Gillebert, Humphreys & Mantini, 2014).
Besides the speed and operator independence, there are several additional advantages of employing a computer-assisted fully automatic infarct detection and localization in NCCT as discussed by Nowinski et al. (2013). Namely, first, the reckoner is able to process multiple density ranges in lodge to observe subtle changes in them. Second, the density changes caused by infarction tin can be accumulated beyond all slices in 3D by the computer, which may facilitate the detection and localization of these changes. Third, the reckoner is able to identify and compare contralateral regions (i.e., symmetrical with respect to the calculated midsagittal plane) which symmetry is critical, especially, when the symmetry in the original images is deteriorated or even completely vanished due to a heavy head tilt (which often happens in scans acquired in the emergency room).
Though in that location be several methods aiming to automate the evaluation of ischemic stroke in MR and CT images, only a few of them address the automatic detection, localization, and/or segmentation of ischemic lesions in NCCT human encephalon scans. Rekik et al. (2012) presented a land-of-the-fine art review in the medical image analysis approaches applied to sectionalisation, prediction, and dynamic evolution modeling of acute/subacute ischemic stroke from CT and MR human, animal and/or synthetic data. Out of 44 papers included in that review, in the category of automated segmentation of human ischemic stroke from NCCT the review lists but vi papers from four centers. These papers are categorized into iii groups: image-based methods (Matesin, Loncaric & Petravic, 2001; Meilunas et al., 2003; Usinskas et al., 2003; Usinskas, Dobrovolskis & Tomandl, 2004), pixel-based methods (Chawla et al., 2009), and atlas-based methods (Maldijan et al., 2001).
Some other review prepared past Mokli et al. (2019) provides a computer-aided imaging analysis in astute ischemic stroke from NCCT, computed tomography angiography, and perfusion imaging. This review lists 26 software applications commercially available from 13 companies for automatic and semi-automatic medical prototype analysis for acute stroke diagnostics, and only 2 of them deal with NCCT ischemic stroke, each employing the ASPECTS score. These are east-ASPECTS® software from Brainomix Ltd. (Oxford, UK) to assess the ASPECTS score and volume of ischemia; and RAPID ASPECTS® from iSchemaView (Menlo Park, USA) to automatically identify and score regions with early ischemic changes using the ASPECTS score. Upwards to engagement, these are the just two commercial products bachelor that are certified for employ in clinical routine. In addition, Borderland ASPECTS from Siemens Healthcare GmbH (Erlangen) is another ASPECTS-based application developed that is not however certified for clinical application.
The goal of this work is to provide: (one) review of the state-of-the-art methods for automatic detection, localization, and/or sectionalisation of ischemic lesions in man encephalon NCCT scans, (2) comparison, evaluation, and nomenclature of the reviewed methods, and (3) recommendation for futurity developments.
Survey methodology
We searched the literature in the examined scope of interest using PubMed and Google Scholar, scanning too in the respective references, selected papers, and related manufactures. We employed the following keywords: "stroke", "ischemic stroke", automated detection", "automated partitioning", "automated localization", and "noncontrast CT".
A number of various methods have been proposed for automated detection, localization, and/or segmentation of ischemic lesions on NCCT in human encephalon scans. We classify these methods into five groups:
-
Image processing and assay-based methods;
-
Brain atlas-based methods;
-
Intensity template-based methods;
-
Stroke Imaging Marker (SIM)-based methods;
-
Artificial Intelligence (AI)-based methods.
Image processing and analysis-based methods
Several image processing and assay-based methods have been proposed for automated handling of NCCT stroke images past employing a variety of techniques including, among others, thresholding, region growing, border detection, textures, wavelets, rule-based skillful systems, classification, and combination of them.
Matesin, Loncaric & Petravic (2001) proposed a rule-based method for segmentation and labeling of ischemic stroke lesions. The method consists of three steps as previously described in Matesin, Loncaric & Petravic (2001): determination of a head symmetry centrality based on moments, seeded region growing to identify multiple regions having uniform brightness, and dominion-based region labeling by using an good system. The rules for identifying the groundwork, skull, brain, and cerebrospinal fluid (CSF) are neighborhood- and intensity-based, and those for an ischemic lesion are symmetry-based. The authors claimed feasibility without presenting any quantitative results.
The group by Meilunas and Usinskas presented iii works. Meilunas et al. (2003) proposed a method based on the contouring of an ischemic stroke region boundary. The method consists of slice filtering, smoothing, and extension of stroke region boundary followed by the calculating of an infarct book.
Usinskas et al. (2003) compared a few methods suggesting that the best viability for ischemic stroke expanse segmentation showed mean, standard deviation, histogram, and grey level co-occurrence matrix methods likewise as a supervised bogus neural network technique.
Later, Usinskas, Dobrovolskis & Tomandl (2004) proposed a texture-based method with an unsupervised classifier. The method uses eighteen unified textural features to segment an ischemic stroke region on images, including joint features from the mean, standard deviation, histogram, and gray level co-occurrence matrix. The method requires thresholding for each image which is non automated. The authors showed an ability to segment an ischemic stroke region without any quantitative assessment.
Przelaskowski et al. (2007) proposed a wavelet-based method. The authors observed that infarction perception tin be improved by data denoising and local contrast enhancement in a multi-calibration domain, and presented a wavelet-based image processing method enhancing the subtlest signs of hypodensity which were often invisible in a standard CT scan review. The method studied on xxx ischemic scans increased the sensitivity of ischemic lesion detection from 12.five% for a standard CT scan preview to 56.3%.
Chawla et al. (2009) proposed a classification-based method in the intensity and wavelet domains. The method detects and classifies a stroke-related aberration into acute infarct, chronic infarct, and hemorrhage by comparing the cerebral hemispheres. The method consists of three steps as previously reported in Chawla et al. (2009): paradigm enhancement and denoising, detection of a brain symmetry line, and classification of abnormal slices. A two-level classification scheme employs an intensity histogram-based comparison to identify chronic and hemorrhagic cases as well equally wavelet energy-based texture information for acute infarct detection. The method was evaluated on half dozen normal and ix stroke patient CT scans resulting in an accuracy of 90%.
Tang, Ng & Chow (2011) and Tang, Ng & Chow (2013) proposed a texture-based method using circular adaptive regions of interest. This method comprises preprocessing (a threshold-based bone and artifact removal), generation of circular adaptive regions of interest, for each region locating by reflection a corresponding round region on the other side of the brain image, and comparing each pair of the circular regions with several texture attributes. These attributes are calculated based on a gray level co-occurrence matrix and they include energy, entropy, inverse difference moment, inertia, prominence, shade, correlation, and variance. The method was tested on 10 acute and 10 chronic ischemic stroke cases resulting in an estimated accurateness of 86.96%.
Boers et al. (2013) presented a method for infarct volume measurement in follow-upward NCCT scans by employing region growing. Afterwards a transmission placement of the seed point in the infarcted hypo-adulterate area, the region growing was repeated for various thresholds in the range of one.5–iv.5 HU (Hounsfield Units) with a step of 0.five HU resulting in 7 segmentations. To avoid the region growing from leaking into the contralateral hemisphere, the midline was used as a limit (that was adamant based on the geometric eye and the most farthermost midsagittal bone or nasal cartilage structures). The algorithm was tested on 34 cases and achieved the Die's Similarity Coefficient (DSC) (Die, 1945) of 34%.
Vos et al. (2013) proposed a classification-based method to find and segment ischemic lesions. The method comprises iii stages as previously described in Vos et al. (2013): pixel nomenclature and lesion candidate localization (via a naive Bayes classifier combined with a tissue homogeneity processing to localize candidates for ischemic lesions), partitioning of the candidate lesions and feature extraction (through a marching cubes algorithm to analyze regional statistics in order to excerpt features based on local and contextual information from the contralateral hemisphere), and aggregation of the extracted features into a likelihood of ischemia (by employing a supervised classifier). The method operation in lesion segmentation accomplished the DSC of 74%.
Tyan et al. (2014) presented an unsupervised feature perception enhancement method for ischemic stroke detection. The method works in four-steps as previously reported in Tyan et al. (2014): preprocessing (utilizing a cubic bend dissimilarity enhancement), encephalon tissue extraction (past applying thresholding, blurring, and morphological operations), meaningful area extraction (through edge detection to identify the stroke area and unsupervised region growing followed by a encephalon area partitioning into eight regions by a horizontal and a vertical line and an elliptic curve determining the border betwixt the gray affair (GM) and white matter (WM)), and infarct regional location (past computing the effulgence in these eight regions, determining areas with the smallest values in comparing to their counterparts, and analyzing their mutual relationships). The method tested on 26 patients demonstrated an increased stroke diagnosis sensitivity of 83% in comparison to 31% when radiologists used conventional diagnostic images.
Ray & Bandyopadhyay (2016) proposed a method based on the textural analysis. The method contains iii modules every bit previously featured in Ray & Bandyopadhyay (2016): preprocessing (by performing noise and artifacts removal), segmentation (past applying image slope magnitude watersheding followed by thresholding), and feature extraction (by dividing an image into four quadrants, computing for each region offset-gild texture measures (the hateful, standard deviation, variation, skewness, kurtosis, and entropy), and selecting an abnormal region based on their values). The results contain a single prototype of an extracted hemorrhage, without any ischemic lesions and quantitative assessment.
Encephalon atlas-based methods
A encephalon atlas is a means for cognition assemblage, presentation, and discovery (Nowinski, 2020a). Electronic brain atlases have potential usefulness in stroke image direction for diagnosis, treatment, and prediction (Nowinski, 2020b). In ischemic stroke detection, a brain atlas enables an automated generation of regions of interest (ROIs).
Maldijan et al. (2001) presented an atlas-enhanced method for identifying potential areas of astute ischemia for center cerebral artery (MCA) stroke. The method first performs prototype preprocessing, including interpolation, scalp striping, normalization, and atlas-based partitioning of the lentiform nucleus and insula. Then, voxels densities in the segmented lentiform nucleus and insula of 1 hemisphere are compared with those in the contralateral side by using the Wilcoxon two-sample rank-sum test. This method, limited to two structures and MCA ischemic stroke, was validated for 15 ischemic stroke patients. Annotation that conceptually, the method is like to the ASPECTS scale with a lower number of ROIs, though delineated and candy automatically.
Nowinski (2020b) proposed a multi-atlas method to detect and localize ischemic and hemorrhagic stroke lesions. It processes the entire brain covered with numerous ROIs that are examined, overcoming the limitation of the ASPECTS-based and Maldijan et al. (2001) methods regarding a pocket-sized number of ROIs. These ROIs are derived from two atlases, an atlas of beefcake and an atlas of claret supply territories, which are in spatial correspondence (Nowinski et al., 2006b). As previously described in Nowinski (2020b) the method calculates the midsagittal plane separating the brain into the right and left hemispheres and is able to handle a large caput tilt often present in emergency room acquisitions (Puspitasari et al., 2009), maps the brain atlases on an NCCT scan through the ellipse-plumbing fixtures atlas-to-scan registration method (Volkau et al., 2012), extracts the ventricular system using a dedicated algorithm for segmentation of the ventricular organization from ischemic stroke NCCT scans (Poh et al., 2012) and removes from the processed images the CSF regions as the CSF density range overlaps with that of infarcts, determines numerous left–right respective ROIs in both hemispheres by employing the atlases, and compares these pairs of the ROIs by means of multiple statistical tests. The method was tested on several ischemic and hemorrhagic cases demonstrating feasibility, and the component algorithms were validated quantitatively on 208 NCCT scans (MSP extraction), 75 multi-modal NCCT, MR, and PET scans (atlas-to-scan mapping), and 102 NCCT stoke cases (ventricular system extraction).
Intensity template-based methods
Gillebert, Humphreys & Mantini (2014) proposed an intensity template-based method to delineate infarcts and hemorrhages. The method comprises two steps every bit previously described in Gillebert, Humphreys & Mantini (2014): patient scan preprocessing (by applying a threshold-based clustering, intensity transformation, MNI (Montreal Neurological Constitute) space normalization, isotropic reslicing, and smoothing) followed by statistical assay for lesion detection (by a voxel-by-voxel comparing of the preprocessed scan with a normal CT browse template developed from 72 non-stroke subjects for defining areas with hypo- or hyper-intense signals). The performance measured by the DSC on 24 astute stroke patients and 72 command subjects with the simulated lesions ranged (depending on the caste of applied smoothing and the level of thresholding) between 52% and 89%.
SIM-based methods
The prototype processing and analysis-based methods typically observe local changes in images, while the SIM-based methods endeavour to capture global density changes caused by an infarct in the scan. Moreover, the SIM-based methods brand detection taking into account the actual patient's values of CSF, WM, and GM. Finally, these methods avoid image processing operations distorting original densities values, such as smoothing or blurring.
Standard SIM-based method
Nowinski et al. (2013) proposed a method for rapid and automatic detection, localization, and volume assessment of ischemic infarcts (including hyperacute, astute, lacunar, and chronic infarcts as well as infarcts with hemorrhagic transformation and leukoaraiosis). The method exploits the fact that an ischemic lesion manifests itself past (i) occupying the density range betwixt CSF and WM, and (ii) redistribution of density globally between the hemispheres. This redistribution, which might be barely observable past the human eye, is captured by the introduced Stroke Imaging Mark (SIM). The SIM determines the infarct spatial range in the centric, coronal, and sagittal orientations past statistically comparing multiple cumulative density distributions calculated for the whole normal and infarcted cerebral hemispheres. As previously described in Nowinski et al. (2013) the method performs in v chief steps: (ane) identify the midsagittal plane (MSP) by applying the algorithm by Puspitasari et al. (2009) and subdivide the encephalon into the correct and left hemispheres; (ii) reformat the originally acquired axial slices through near neighbor interpolation (to avoid irresolute the original density values) such as to be precisely perpendicular to the MSP, every bit typical stroke acquisitions usually do not produce exactly symmetrical images; (3) calculate for each hemisphere the patient-specific density ranges of CSF, WM, and GM employing the algorithm past Gupta et al. (2010); (4) compute the spatial extent of the ischemic infarct by determining its range in each centric, coronal, and sagittal orientations through the SIM; and (5) calculate 2 cuboidal regions inner and outer that localize the ischemic infarct in 3D.
The SIM is composed of three components and information technology is computed every bit:
SIM = P-ratio * N-ratio / MDV,
where:
-
P-ratio denotes a percentile difference ratio in several small subranges of the entire density distribution;
-
N-ratio denotes a ratio of voxels count in two density bands within the brain parenchyma;
-
MDV is the median density value.
As previously explained in Nowinski et al. (2013) the SIM is computed for every image in each hemisphere, and each axial, coronal, and sagittal orientation. The SIM is adaptive to diverse manifestations of the infarcted region by employing a prepare of parameters determining the density subranges resulting in 54 diverse combinations (SIM plots). The combination that achieves the maximum SIM difference across the normal and infarcted hemispheres is taken as the most significant effect past applying the Wilcoxon rank-sum test. The spatial extent of the infarct is adamant by the starting and ending locations of the intersection points of the SIM plots for both hemispheres enclosing the largest number of the consecutive slices. The 5th and 95th percentiles of the distribution of the starting and ending intersections are considered the lesion localization limits, and when calculated for the axial, coronal, and sagittal orientations they demarcate two bounding boxes, the inner and the outer, that localize spatially the infarct.
As described earlier by Nowinski et al. (2013) the method was quantitatively validated on 576 clinically confirmed strokes, each with a single NCCT browse. The scans were caused at four centers in ii countries. The scans consisted of cadre scans of 322 "pure" astute ischemic infarcts (i.e., without whatever other noticeable pathology), 36 lacunar infarcts, 17 hemorrhagic transformations, 104 ischemic infarcts jointly with chronic infarcts, and 70 acute ischemic infarcts along with leukoaraiosis. Out of the total of (104+70) chronic infarcts and leukoaraiosis cases, 27 scans had ischemic infarcts along with both leukoaraiosis and chronic infarcts. The fourth dimension afterward the onset of symptoms at conquering was available for 532 scans, and it spanned from one.five h (hours) to 72 h for 450 scans and higher up 72 h for 82 others. These scans were divided into 3 h, 3< to 8 h, and >8 h later on the onset of symptoms. In addition, 21 NCCT hyperacute cases (between i.five h and 7 h) with additional follow-upward NCCT imaging were used for early stroke detection.
As previously reported in Nowinski et al. (2013) the SIM method matched 100% good's infarct detection achieving 99.8% inner localization specificity and 93.3% outer localization sensitivity when leukoaraiosis cases, chronic infarcts, and infarct volumes <ii cm3 were excluded. For all the cases while omitting infarct volumes <two cm3, this detection accuracy lowered to 95.7%. For any case, detection accurateness further reduced to 83.2%. Early detection accurateness (≤3 h) was 78.4% and this accuracy increased with the raise of the time after the onset of stroke symptoms from 78.4% (≤three h) to 80.1% (iii< to ≤ 8 h) to 87.9% (8< to ≤ 72 h). In addition, the SIM method also detected all 21 early ischemic infarcts (of which xv were overlooked past stroke neuroradiologists).
Modified SIM-based method
The promising results obtained for early ischemic stroke detection encouraged us to farther study the SIM to meliorate its performance for hyperacute ischemic stroke. Two quantities were studied in the SIM formula: (1) parameter selection and their value setting every bit well every bit (2) its components (Gomolka et al., 2016).
In the standard (original) SIM method the P-ratio was sampled with six density ranges and the N-ratio with nine density ranges giving rise to 54 parameter combinations. This parameter setting resulted in quick infarct detection and localization in 7 south. The modified SIM method employed a wider spectrum of density ranges in terms of their span (from five to 40 HU), coverage, and numbers, including finer ranges, resulting in a total of 168 parameter combinations.
These parameters were modified and examined simply in the P-ratio equally the report showed that the N-ratio was optimally formulated and the MDV was excluded from the modified SIM for efficiency as its average value was similar in the infarcted and normal hemispheres amidst all the early scans (indicating that early infarction causes changes in density distribution rather than forming hypodense areas).
As previously reported in Gomolka et al. (2016) the modified SIM method was evaluated on 70 early (for the detection) and 70 follow-upwardly (to prepare of the gold standard) ischemic stroke scans from two centers. The all-time performance was obtained for the P-ratio including seven percentile subranges within the range of 35th-75th percentile achieving a 76% ischemic hemisphere detection charge per unit and 54% sensitivity in spatial localization of hyperacute ischemia.
AI-based methods
Bogus Intelligence (AI) is divers in the Merriam-Webster lexicon (https://www.merriam-webster.com/) as: "(ane) a co-operative of computer science dealing with the simulation of intelligent behavior in computers, and (2) the capability of a car to imitate intelligent human beliefs". Machine learning is one form of artificial intelligence that "is devoted to edifice algorithms that allow computers to develop new behaviors based on experience" (https://www.merriam-webster.com/). In other words, automobile learning develops algorithms enabling computers to acquire from existing data without explicit programming.
Automobile learning methods are subdivided into supervised learning and unsupervised learning. In supervised learning, the algorithms are first trained past employing some existing "gilt standard" or "ground truth"; in the considered application this is a collection of brain NCCT scans classified into infarcted versus no infarcted. Supervised learning methods include, among others, linear regression, back up vector machines, determination trees, random conclusion forests, and k-nearest neighbors (m-NN) algorithm (classifiers for ischemic stroke lesion segmentation are reviewed and compared by Maier et al. (2015)). In contrast, unsupervised learning attempts to discover previously unknown classes, patterns, and/or structures in the data with no given classification nor previous training. Unsupervised learning methods include, among others, k-means clustering, mixture models, and subconscious Markov model. In radiology at present, the dominant type of car learning algorithm is the artificial neural network (ANN) which is a cluster of interconnected nodes (Dreyer & Geis, 2017). An ANN with multiple layers of interconnected nodes with representation learning is termed deep learning. Deep learning has recently become the principal form of car learning considering of a convergence of theoretic advancements, openly bachelor computer software, and hardware with adequate computational power (Zaharchuk et al., 2018). Deep learning through computationally efficient convolutional neural networks (CNNs) is well-suited for imaging (Zaharchuk et al., 2018). The CNNs require a large amount of preparation information to avoid overfitting, and once the network parameters have converged an additional training step is performed to fine-tune the network weights. To reduce the amount of training data and to produce more precise segmentation of biomedical images, U-net architecture is developed based on CNNs (Ronneberger, Fischer & Brox, 2015).
Rajini & Bhavani (2013) proposed a detection method past employing texture features combined with various car learning methods. The method consists of v stages: preprocessing, segmentation, brain midline tracing, extraction of 14 texture features (using a gray level co-occurrence matrix between the left and right hemispheres), and nomenclature (by a binary classifier). As reported earlier past Rajini & Bhavani (2013) the method was validated quantitatively on 15 ischemic cases and, to distinguish an ischemic from normal hemisphere by applying a support vector machine, k-nearest neighbors, artificial neural network, and decision tree classifiers, it accomplished the accuracy of 98%, 97%, 96%, and 92%, respectively.
Sales Barros et al. (2019) proposed an infarct segmentation method utilizing CNN deep learning. The goal was to segment an infarct to calculate its volume in follow-upward NCCT scans acquired between 12 h and 2 weeks after stroke onset. The method has two steps as previously described in Sales Barros et al. (2019): preprocessing to segment the intracranial region (through thresholding, region growing, and morphological operations) and CNN-based infarct segmentation (with the CNN compages with two convolutional layers followed by ii fully continued dense layers, each dense layer with 256 nodes).
For validation 396 NCCT stroke scans were employed to test sectionalization functioning, and boosted 570 scans for training, and 60 for parameter fine-tuning. Patients with anterior circulation stroke were selected for this study, which is its major limitation. A unmarried trained CNN achieved for all tested 396 patients the DSC of eighteen%. As this value is depression, the scans were additionally divided into three infarction classes with fixed thresholds: astringent of [14, 22] HU, intermediate of [22, 32] HU, and subtle of [32, 44] HU. Then by employing three CNNs, the respective values of the DSC were 78% for the severe form with 67 cases, 61% for the intermediate with 204 cases, and 37% for the subtle class with 125 cases.
Kuang et al. (2019) proposed a deep learning method to automate the ASPECTS score based on texture features extracted from each ASPECTS region to subsequently train a random forest (RF) classifier. The classifier was trained for 157 cases. The method tested on 100 patients resulted in a sensitivity of 66.2%, specificity of 91.eight%, and area under the curve of 0.79. This operation was further improved when the ASPECTS was dichotomized (>4 and ≤4) achieving a sensitivity of 97.8%, specificity of 80%, and area under the bend of 0.89.
Three deep learning approaches have been proposed past the group of Kuang, Menon, and Qiu to segment follow-upwardly NCCT scans to measure post-handling cerebral infarct volumes for evaluating the effectiveness of endovascular therapy of acute ischemic stroke patients.
Kuang, Menon & Qiu (2019a) presented an infarct segmentation method that combines machine learning exploiting cascaded RF and interactive sectionalization. The method includes iii major steps as reported by Kuang, Menon & Qiu (2019a): expert initialization, RF learning and classification (with a two-stage grooming and testing classifier), and convex optimization-based segmentation. The initialization stride requires the user's input knowledge to pre-label some voxels in the infarcted region and background on a few axial slices aiming to lessen the detected false positives (making in this way the method semi-automatic). A cascaded RF learning is applied to allocate each voxel into normal or ischemic, and to calculate an infarct probability map. 4 kinds of features are extracted: intensity, statistical information in the local region, the symmetric difference compared to the contralateral side (past using paradigm symmetry), and the spatial probability of infarct occurrence. These features are input into the RF to train a first-phase classifier whose coarse results of sectionalization are employed to train a 2d-stage fine classifier with fivefold cross-validation. The RF estimated infarct probability map calculated by the 2d-stage classifier with user input knowledge is afterward included in a convex optimization office to get the concluding segmentation. One hundred stroke patients were used in this study, of which 70 scans for evaluation and thirty for grooming achieving the DSC of 79%. The method considerably outperformed some other AI methods, including the RF-based methods and CNN-based U-net.
Another method proposed by Kuang, Menon & Qiu (2019b) is based on dense Multi-Path Contextual Generative Adversarial Network (MPC-GAN). It makes utilise of a dense multi-path U-Net equally a generator regularized by a discriminator network. The generator and discriminator input contextual data, such as bilateral intensity divergence, infarct location probability, and distance to CSF. The MPC-GAN network was trained on sixty patients, fine-tuned on ten patients, and thirty patients were used for validation yielding the DSC of 72.six%. The MPC-GAN method outperformed some land-of-the-art segmentation methods, such as the U-Internet, U-Net based GAN, and RF-based division method.
Kuang, Menon & Qiu (2019c) presented a deep learning-based semi-D-net method for the simultaneous division of infarcts and hemorrhages. The method integrates network learned semantic information, local image context, and user initialized prior to a multi-region contour evolution scheme, which subsequently is globally optimized by a convex relaxation technique. The method besides introduces a D-Unet architecture that follows that of U-Net, and semi-D-Unet that additionally requires user input knowledge. As reported earlier by Kuang, Menon & Qiu (2019c) a quantitative evaluation using 30 cases yielded the hateful DSCs of 67.4% for ischemic infarct, 65.3% for hemorrhage, and 72.five% for both. Post-processing using multi-region evolution and the introduction of user interactions greatly improved accuracy. The proposed method outperforms other deep-learning methods including the U-net, D-net, demi RF, and semi-U-cyberspace.
Discussion
The give-and-take covers a comparison of the individual methods and evaluation of their advantages and limitations, a characterization of the groups of the methods, a proposal of a new classification of the methods, and equally a conclusion a recommendation for future development.
The proposed methods are commonly a combination of diverse techniques and approaches, and the principal approaches are various image processing and assay techniques, assessment of disproportion betwixt the left and correct cerebral hemispheres, ROI-based assay, and AI-based classifiers.
The reviewed methods are summarized and compared in Table i taking into account: author(s), type of method along with techniques, use of brain symmetry (with respect to the prototype or by calculating the MSP), ROI-based analysis (along with the number of employed ROIs), type of stroke/infarct (ischemic, hemorrhagic, hyperacute ischemic, astute ischemic, chronic ischemic, follow-up ischemic, lacunar, infarct with hemorrhagic transformation, and infarct with leukoaraiosis), and validation (including the number of stroke cases and availability of quantitative validation).
Table 1:
Comparison of automated methods for ischemic infarct detection, localization , and segmentation in NCCT scans of the man encephalon.
| Author(s) | Method (group and techniques) | Left/correct symmetry | ROI assay | Type of stroke | Validation | |||
|---|---|---|---|---|---|---|---|---|
| Y/N | MSP | Y/N | Number of ROIs | Number of stroke cases | Quantitative assessment | |||
| Matesin, Loncaric & Petravic (2001) | IPA; rule-based, region growing | Y | Due north | N | NA | I | NA | Northward |
| Meilunas et al. (2003) | IPA; contour-based, filtering, smoothing | N | N | N | NA | I | NA | North |
| Usinskas et al. (2003) | IPA; mean, standard deviation, histogram, co-occurrence matrix | N | N | Northward | NA | I | NA | N |
| Usinskas, Dobrovolskis & Tomandl (2004) | IPA; texture-based, thresholding (not automated) | N | N | N | NA | I | NA | N |
| Przelaskowski et al. (2007) | IPA; wavelet-based | North | Northward | N | NA | I | xxx | Y |
| Chawla et al. (2009) | IPA; intensity-based, wavelet-based, two-level classification | Y | North | N | NA | I-a, I-ch, H | 9 | Y |
| Tang, Ng & Chow (2011); Tang, Ng & Chow (2013) | IPA; texture assay, radius variable ROIs | Y | N | Y | Variable | I-a, I-ch | 10 astute, 10 chronic | Y |
| Boers et al. (2013) | IPA; region-growing with multiple thresholds | N | Y | N | NA | I-fu | 34 | Y |
| Vos et al. (2013) | IPA: Bayes classification, marching cubes segmentation, supervised nomenclature | Y | NA | North | NA | I | NA | Y |
| Tyan et al. (2014) | IPA; edge detection, region growing, blurring | Y | N | Y | 8 | I | 26 | Y |
| Ray & Bandyopadhyay (2016) | IPA; textural analysis, watersheding, thresholding | Y | Northward | Y | iv | I | 0 | North |
| Maldijan et al. (2001) | BA; limited to MCA, interpolation, normalization | Y | Due north | Y | 2 | I | 15 | Y |
| Nowinski (2020b) | BA; two atlases, atlas individualization, ventricular organisation extraction, statistical tests | Y | Y | Y | Many | I, H | Several | Y (component algorithms) |
| Gillebert, Humphreys & Mantini (2014) | IT; normalization, smoothing, statistical assay | N | N | Due north | N | I, H | 24 | Y |
| Nowinski et al. (2013) | SIM; original, CSF/WM/GM calculation, density sampling | Y | Y | Northward | NA | I-ha, I-a, I-ht, I-ch, I-lac, I+la | 576 | Y |
| Gomolka et al. (2016) | SIM; modified, CSF/WM/GM calculation, density sampling | Y | Y | Northward | NA | I-ha | 70 | Y |
| Rajini & Bhavani (2013) | AI; texture features, classifiers (support vector car, 1000-nearest neighbors, artificial neural network and decision tree) | Y | N | N | NA | I | fifteen | Y |
| Sales Barros et al. (2019) | AI; segmentation, region growing, morphological operations, deep learning with CNN | Northward | N | N | NA | I-fu | 396 (67, 204, 125 for individual classes) | Y |
| Kuang et al. (2019a) | AI; texture features and RF classifier for ASPECTS | N | N | Y | x | I | 100 | Y |
| Kuang, Menon & Qiu (2019a) | AI; cascaded RF with interactive segmentation | Y | N | N | NA | I-fu | 70 | Y |
| Kuang, Menon & Qiu (2019b) | AI; dumbo MPC-GAN | N | N | N | NA | I-fu | xxx | Y |
| Kuang, Menon & Qiu (2019c) | AI; semi-D-net with user initialized prior | N | N | N | NA | I-fu, H | xxx | Y |
Characterization of groups and evaluation of methods
The reviewed methods enable automatic detection, localization, and/or partition of ischemic lesions. An advantage of having the infarct segmented is that information technology then can hands exist quantified, including calculation of its book, which is an important radiologic effect measure out of the effectiveness of endovascular therapy. However, ischemic infarcts on NCCT images show neither homogenous density nor abrupt edges, endure from a low bespeak to noise ratio, and interfere with chronic infarcts and leukoaraiosis, among others, as illustrated in Fig. i. Therefore, their accurate segmentation is difficult for real clinical data, if possible at all for acute and hyperacute cases. A more practical approach seems to provide infarct detection cum localization with the infarct book estimated statistically past applying, for instance, a method presented by Nowinski et al. (2013). Alternatively, infarct segmentation is carried out on follow-up NCCT images as presented by Sales Barros et al. (2019), Kuang, Menon & Qiu (2019a), Kuang, Menon & Qiu (2019b) and Kuang, Menon & Qiu (2019c)).
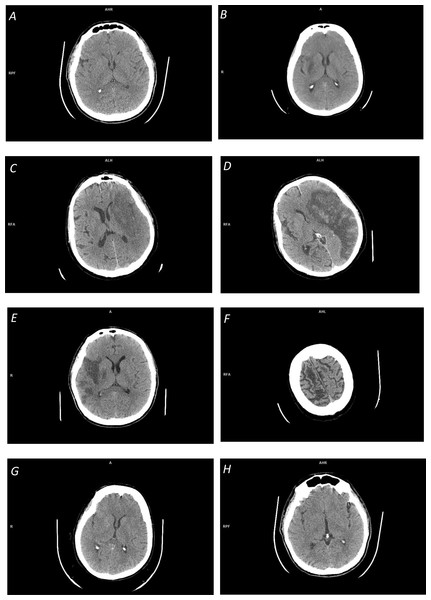
Figure i: Illustration of infinite-dependent ischemic stroke changes on NCCT scans:
(A) Early ischemic stroke with invisible changes; (B) focal inhomogeneous ischemic lesion in the right basal ganglia with a fuzzy edge; (C) focal large ischemic presence in the left MCA territory with a partly fuzzy ischemic lesion at its posterior edge; (D) focal large ischemic presence in the left MCA territory with a articulate ischemic lesion border (along with hemorrhagic transformation); (Eastward) multi-focal ischemic lesion of the correct MCA territory; (F) overlapping density cum a fractional book issue in cerebrospinal fluid spaces; (Thou) distributed ischemic presence, obscuring of basal ganglia density (in the right lentiform nucleus) and loss of distinction between gray and white matter; and (H) distributed early ischemic presence, sulcal effacement in the right fronto-parietal region.The majority of the reviewed methods are based on prototype processing and analysis. Some of these methods apply thresholding, edge detection, and region growing for infarct segmentation, however, the outcome of these operations is questionable (particularly for real clinical information). Get-go, these operations are sensitive to racket. Second, region growing is not able to span gaps betwixt multiple infarcted areas (Boers et al., 2013), run across Fig. 1E. Third, the ischemic lesions are not regions with uniform intensities (see Figs. 1B, 1C) just prove smaller decreases on the borders and larger decreases towards the center of the lesion (Rekik et al., 2012), so these methods are not able to conspicuously decide the lesion boundary. And quaternary, the density range of ischemic lesions overlaps with that of CSF (see Fig. 1F), and because of this and a fractional volume effect in the area around CSF, the latter may be falsely included in the segmented region.
Several methods employ smoothing equally a preprocessing pace, which may have an impact on a precise delineation of lesion boundaries (Seghier et al., 2008). Moreover, larger smoothing values penalize the detection of small lesions by blending them with the surrounding tissues.
To cope with some of these shortcomings, more than powerful approaches take been proposed in image and wavelet domains with a battery of operations, a plurality of texture attributes (such every bit free energy, entropy, moments, inertia, shade, prominence, correlation, skewness, kurtosis, and variance), and diverse classifiers. These approaches are enhanced by a left–right hemisphere comparison and ROI processing and analysis.
It likewise shall be noted that some authors claim their methods to exist automated, merely they are really semi-automated requiring human interaction, such as a seed signal placement (Boers et al., 2013) or a threshold setting (Usinskas, Dobrovolskis & Tomandl, 2004).
The brain atlases are useful ways for prototype partitioning by the individualized atlas (or atlases) superimposed on a scan, enabling in this way an ROI-based analysis and a comparison of the right and left cognitive hemispheres (Nowinski, 2020a). The number of prototype partitions varies from a few for some office of the encephalon (similar only two in the MCA territory (Maldijan et al., 2001)) to many delineated past multiple complementary whole-encephalon atlases (Nowinski, 2020b). A fast and automated atlas-to-scan mapping is critical in stroke epitome management and we have developed earlier suitable methods for this purpose (Nowinski et al., 2006a; Volkau et al., 2012).
The intensity template based-methods exploit a mutual arroyo for abnormality detection past comparison a patient brain to some reference, neurologically normal command encephalon. To enable comparison with a plurality of control brains, an intensity template must be created by normalizing them to the aforementioned stereotactic space. Then, the stroke patient browse is normalized to the template and statistically compared to it on a voxel-by-voxel basis aiming to identify typical areas. In contrast to brain atlas-based methods, this grouping of methods can simply be employed to stroke patient scans that have the same image modality equally the intensity template. In the example of NCCT, the patient scan-template comparison aims at identifying regions with hypo- or hyper-intensity indicating a suspected ischemic or hemorrhagic lesion, respectively. An advantage of this approach is that it handles simultaneously both ischemic and hemorrhagic stroke; in fact, Gillebert, Humphreys & Mantini (2014) claim that their intensity template-based method was more specific in distinguishing hemorrhage than ischemia. A limitation of this approach is that ischemic lesions, as discussed above, exercise non demonstrate uniform intensities and overlap with other regions. The same holds for hemorrhagic lesions whose properties were studied on 289 NCCT hemorrhagic stroke scans past Nowinski et al. (2014a). These lesions bridge a 25–88 HU range, in contrast to other studies compared in (Nowinski et al., 2014a) indicating a much narrower range, such as threescore-80 HU (New & Aronow, 1976). Moreover, hemorrhagic lesions substantially overlap with GM and to some extent with WM density ranges.
Noesis aggregation, limited to averaging of normal encephalon scans in the intensity template-based methods, has a much wider potential in atlas-assisted approaches. Nosotros accept created a probabilistic stroke atlas aggregating radiologic (imaging) and neurologic (numerous parameters) knowledge in the population (Nowinski et al., 2014b). The atlas originally employed for the prediction of ischemic stroke outcomes (by mapping imaging into neurologic parameters) can be potentially employed to enhance lesion detection (by applying a reverse mapping).
The image processing and assay-based and the intensity template-based methods exploit local changes caused by an ischemic lesion, while the encephalon atlas-based methods examine the changes occurring in the atlas-defined ROIs. In dissimilarity to these three groups of methods, the SIM-based methods capture the patient-specific density distribution changes globally, both in the infarct itself and the surrounding information technology parenchyma. This characteristic is peculiarly beneficial when the infarct is in the hyperacute stage, so when its focal hypodensity (see Fig. 1A) and/or whatsoever distributed presence (run across Figs. 1G, 1H) might be very subtle or fifty-fifty inappreciably discerned by the human middle. The modified approach has demonstrated that the finer density sampling with a larger number of density sub-ranges improves infarct detection in the hyperacute stage. This study too confirmed that the average density of the normal and infarcted hemisphere for hyperacute cases are the same, indicating that the hyperacute stroke detection approaches based on ischemic lesion features themselves will probably fail.
As previously reported past Nowinski et al. (2013) the usage of 2 different infarct localization 3D bounding boxes that are superimposed on the candy scan accumulates advantages of the high sensitivity of the outer localization bounding box and the high specificity of the inner localization bounding box. Consequently, the inner localization bounding box marks the infarcted region (meaning it works like a cursor) and the outer localization bounding box estimates the infarct extent. It is worth noting that this approach tin guess the book of an ischemic infarct.
AI has been changing our world in many aspects, and its impact will inevitably grow in the years to come up. In detail, deep learning has shown remarkable promise in solving many bug in calculator vision, natural linguistic communication processing, and robotics (LeCun, Bengio & Hinton, 2015) with various neural network architectures proposed, including U-Net, D-Unet, ReLU, ConvNet, ResNet, ConsNet, and MPC-GAN. Neural networks are a very intensive surface area of research. For instance, on Google Scholar under term "neural network" there are 2.7 million results and under "convolutional neural network" 456 thousand results. More than specifically, under "stroke convolutional neural network" at that place 25.iv thousands results, and under "acute ischemic stroke convolutional neural networks" 18.seven thousand results. In particular in stroke management, Feng et al. (2018) merits that deep learning techniques, because of their speed and power, will become an increasingly standard tool for stroke experts. Furthermore, Maier et al. (2015) compared several classification methods for ischemic stroke lesion sectionalisation, although for MR scans, and ended that high-level machine learning techniques, such as CNNs and random decision forests, lead to significantly better segmentation results compared to the rather simple classification methods such as kNN, Gaussian naive Bayes, and generalized linear models.
The papers reviewed here , however, practise non demonstrate the fulfillment of these promises in the considered area all the same. The method by Rajini & Bhavani (2013) employs heavy image processing components combined with several simple nomenclature methods tested on a low number of fifteen patients.
The methods proposed in Sales Barros et al. (2019), Kuang, Menon & Qiu (2019a) Kuang, Menon & Qiu (2019b) and Kuang, Menon & Qiu (2019c) use advanced deep learning techniques for follow-up NCCT scans which are easier for processing than hyper-acute and astute cases. The CNN-based method by Sales Barros et al. (2019) tested on a large dataset of 396 cases yielded the DSC of 34%. For the belatedly cases, the DSC was increased to 78%. This approach uses stock-still thresholds for density ranges, in dissimilarity to the SIM-based methods that utilize patient-specific density ranges for the calculated CSF, WM, and GM.
The methods proposed by Kuang, Menon & Qiu (2019a), Kuang, Menon & Qiu (2019b) and Kuang, Menon & Qiu, (2019c) employ various network architectures tested on a relatively small number of cases yielding a moderate functioning. This operation was substantially improved past introducing high-level human noesis to drive the segmentation (which makes the methods semi-automatic).
The existing powerful deep learning techniques are inferior to the SIM-based methods (even when tested on easier data). The reason for this is that the SIM-based methods capture and process the overall changes in both the infarcted region and the parenchyma for the entire density spectrum along with the employment of patient-specific ranges, while the deep learning methods seem to focus on learning the backdrop of the infarcted regions simply and ofttimes utilize fixed values of parametres.
It shall exist noted that the AI methods can be used optionally with preprocessing (such every bit (Rajini & Bhavani, 2013) to extract texture features before supervised classification) and/or with postprocessing (such equally Kuang, Menon & Qiu (2019c) to perform multi-region evolution and to practise image median filtering for noise elimination in segmentation of stroke lesions from CT perfusion images (Liu et al., 2019)). Preprocessing, postprocessing, and loftier-level domain cognition profoundly meliorate the accuracy of AI-based methods as illustrated past Rajini & Bhavani (2013) and Kuang, Menon & Qiu (2019c).
Nearly of the epitome processing and assay-based methods and the encephalon atlas-based methods exploit the comparison of values in the left and right whole hemispheres or some parts of them, ordinarily the MCA territories. This spatial left–right correspondence is obtained in various means, namely, by image partitioning into quadrants (Ray & Bandyopadhyay, 2016), using paradigm symmetry (Chawla et al., 2009), through individualized atlas (Nowinski, 2020b), brain midline tracing (Rajini & Bhavani, 2013), or past automatic adding of the MSP in 3D (Nowinski et al., 2013). Approaches based on paradigm symmetry or ROI reflection (Tang, Ng & Chow, 2013) will not be able to handle existent clinical cases as the left–correct hemisphere symmetry is generally absent-minded in clinical stroke scans. Moreover, standard algorithms for adding of the MSP may ofttimes neglect for some stroke NCCT scans acquired in the emergency room due to a large brain tilt. Therefore, nosotros accept adult a defended algorithm for MSP calculation to robustly handle stroke cases (Puspitasari et al., 2009).
Some of the image processing and analysis-based and the brain atlas-based methods employ an ROI assay. The number of ROIs varies across methods, namely, 2 (Maldijan et al., 2001), four (Ray & Bandyopadhyay, 2016), eight (Tyan et al., 2014), x (ASPECTS), and multiple when generated by brain atlases (Nowinski, 2020b). The number of ROIs per method may be fixed (equally in the abovementioned methods) or be variable equally in (Tang, Ng & Chow, 2013). The shape of ROIs is predefined by the way of image segmentation (Tyan et al., 2014; Ray & Bandyopadhyay, 2016), results from the constructed encephalon atlases (anatomical structures and vascular territories) or is given (e.1000., circular ROIs in Tang, Ng & Chow (2013). The size of ROIs during processing is mostly fixed or variable as in Tang, Ng & Chow (2013) being circular with an adjustable radius.
The telescopic of validation varies amidst the reviewed methods, with some with no quantitative validation at all and the majority of them with a small-scale number of stroke cases. Until today to our best knowledge, the standard SIM method by Nowinski et al. (2013) tested quantitatively on 576 stroke cases from four centers in two countries is the most thoroughly validated method in terms of the number of stroke cases and their variety. The nearly highly validated method of the AI-based group is that of Sales Barros et al. (2019) with 396 cases, though resulting in a very depression functioning (the DSC of 18%). The highest number of proposed methods belong to the image processing and assay-based grouping, and the about highly validated method of this group used 30 cases (Przelaskowski et al., 2007).
Finally, the reviewed methods differ in their novelty and intellectual property. Namely, three methods are patented, that of Tang, Ng & Chow (2011) holds one U.s.a. patent (Tang, Ng & Chow, 2013), and the methods by (Nowinski et al., 2013; Nowinski, 2020b) are based on 4 US patents and several US patent applications pending (out of our 17 US stroke-related patents listed in Nowinski, 2020b).
This comparing of methods has limitations in terms of completeness, performance measures, task performed, criteria for method grouping, and testing browse selection. Although we have tried our best to have this review every bit complete as possible using PubMed and Google Scholar, there might be some relevant works not listed there. Dissimilar authors apply diverse performance measures, which hinder a fair comparison of methods. Infarct detection and infarct partitioning are two different tasks. Infarct detection uncovers the presence of whatsoever infarcted region (possible with its localization) while infarct sectionalization is performed mainly to quantify the cerebral infarct book every bit an important result measure. Moreover, the bulk of infarct segmentation methods are tested on follow-up scans, where ischemic infarcts are more than prominent than in acute cases. A specific method ofttimes is a combination of various techniques, and so the groups of methods may overlap and the presented grouping of them is not unique. For instance, several image processing and analysis-based methods employ classifiers, which are part of epitome analysis and too of AI; conversely, some AI methods employ epitome processing techniques for pre- and mail service-processing.
Finally, our experience shows that probably the about disquisitional for a fair comparison is the selection of a dataset for testing. NCCT ischemic stroke scans differ from existence easy for processing to difficult; from pure ischemic stroke to stroke with hemorrhagic transformation, chronic infarct, and/or leukoaraiosis; from modest lacunar infarcts to large infarcted areas; and from hyper-astute stroke (i.v h from the stroke onset) to tardily stroke (several weeks after the stroke onset) to chronic infarcts (several months after the stroke onset). This difference in data selection for the same method was demonstrated, for instance, by Sales Barros et al. (2019), where the performance measured by the DSC ranged from 37% for the subtle infarct form to 78% for the severe infarct class; every bit well as by Nowinski et al. (2013) for the detection accuracy raising from of 78.four% for cases with ≥iii h from the stroke onset to 87.9% for cases with 8<to ≥ 72 h from the stroke onset. Time-dependent ischemic stroke changes over a two-month menstruum in terms of appearance and HU density characteristics for the same patient are illustrated in Fig. 2. Annotation the growing prominence of the ischemic lesion and the decreasing mean density from 30 HU (1.five h) to 23 HU (1 day) to 18 HU (one week) to 13 HU (one month) to 10 HU (two months.)
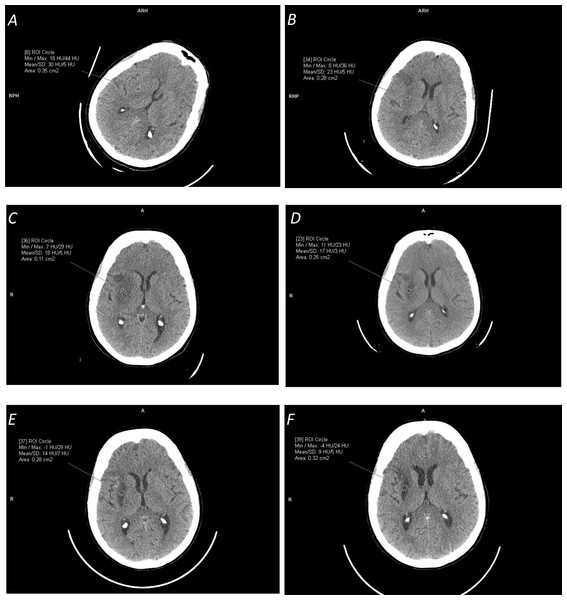
Effigy two: Illustration of time-dependent ischemic stroke changes in terms of advent and Hounsfield Unit (HU) density characteristics (measured in the marked circular regions) for the same patient for the stroke onset after:
(A) i.5 hours (with [18,44] HU range); (B) 24 hours (with [viii,36] HU range); (C) 1 week (with [7,29] HU range); (D) ii and a one-half weeks (with [eleven,23] HU range); (Eastward) i month (with [14,7] HU range); and (F) 2 months (with [-iv,24] HU range). Annotation the alter in the appearance of the ischemic lesion and its continuously decreasing density from the hateful value of 30 HU to nine HU.Classification of methods
Rekik et al. (2012) divided methods for ischemic stroke image management into four groups: pixel and voxel-based classification (the well-nigh mutual), prototype-based segmentation, atlas-based segmentation, and deformable model-based sectionalization. In Section 2 nosotros take classified the reviewed methods into v groups: prototype processing and analysis-based, brain atlas-based, intensity template-based, SIM-based, and AI-based. Additionally to this nomenclature, In Tabular array 1 some supplementary criteria are applied including a left–right hemisphere symmetry, employment of ROI-based assay, and type of validation.
In fact, these divisions are somehow arbitrary, as the methods typically use a bombardment of diverse techniques ranging from prototype processing and analysis to atlas-assisted processing to statistical analysis to machine cognition.
Therefore, we suggest here some other classification scheme that is more than related to a strategy of ischemic infarct management than to a particular technique. And so, from a standpoint of prototype scope, one strategy is to provide local or regional processing and analysis to detect ischemic lesion changes, and on the other manus, the whole brain scan tin can be processed to detect ischemic changes. From a standpoint of image handling, the paradigm spatial extent tin can be sampled and candy or its density range can be sampled. Hence, this nomenclature can be considered every bit a 2 × 2 matrix with local versus global processing and analysis, and density versus spatial sampling. And then, for instance, the SIM-based methods belong to the global, density sampling category with multiple density bands; the atlas-based methods to spatial sampling category that can be regional or global; and the intensity template-based methods to the local category every bit it detects in the lesion expanse (they also may be considered as a marginal instance of density sampling with a single band). The spatial sampling rate may range from low (Maldijan et al., 2001), to medium (ASPECTS), to loftier (Nowinski, 2020b). The density sampling rate is also variable, considered existence medium in the standard SIM-based method and high in the modified SIM-based method.
Conclusion
Future studies are necessary to develop more efficient methods and we recommend two directions for method evolution. One is AI, equally it is considered radiology's next frontier (Dreyer & Geis, 2017) and deep learning techniques are supposed to become a standard tool for the modern stroke specialist (Feng et al., 2018), although the results demonstrated so far in ischemic infarct detection and segmentation from NCCT using the AI methods are moderate. Now, the SIM-based methods outperform the state-of-the-art deep learning techniques, because they procedure the overall changes in the infarcted region and the parenchyma for the unabridged density spectrum with patient-specific density ranges, while the deep learning methods seem to focus on learning in infarcted regions only with fixed parameter values.
Another direction is to combine the advantages of the SIM-based method (Nowinski et al., 2013) and the multi-atlas guided method (Nowinski, 2020a) enhanced by the probabilistic stroke atlas (Nowinski et al., 2014b). In other words, the future development shall be directed toward the combination of the global methods with a high sampling both in space and density forth with the employment of merged radiologic and neurologic data.
Additional Data and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Wieslaw 50. Nowinski conceived the concept, prepared the tabular array and contributed to figures, authored drafts of the paper, approved the final version.
Jerzy Walecki and Katarzyna Sklinda performed the experiments, authored or reviewed drafts of the paper, and approved the terminal draft.
Gabriela Półtorak-Szymczak and Bartosz Mruk analyzed the information, prepared figures and/or tables, and approved the final draft.
Information Availability
The following information was supplied regarding data availability:
This is a review article; at that place is no raw data.
Funding
The authors received no funding for this piece of work.
References
-
ECASS Investigators, Thrombolysis with alteplase three to 4.five hours after astute ischemic stroke. New England Periodical of Medicine 359 :1317-1329
-
Deep learning. Nature 521 :436-444
-
Multi-scale deep convolutional neural network for stroke lesions division on CT images. In: Crimi A , Bakas S , Kuijf H , eds. Encephalon lesion: glioma, multiple sclerosis, stroke and traumatic brain injuries, Lecture notes in estimator science, vol. 11383. Cham: Springer International Publishing. 283-291
-
Automatic detection, localization and volume estimation of ischemic infarcts in noncontrast CT scans: method and preliminary results. Investigative Radiology 48 (nine):661-670
-
Automatic partitioning of cerebral infarcts in follow-up computed tomography images with convolutional neural networks. Journal of Neurointerventional Surgery
-
Deep learning in neuroradiology. American Journal of Neuroradiology 39 (ten):1776-1784
Source: https://peerj.com/articles/10444/
Posted by: leverettfainizind.blogspot.com


0 Response to "Anxiety Disorders Show Changes In Cerebral Hemisphere Symmetry In Which Scan?"
Post a Comment